April 2019 – Issue 71
Many Wagyu breeders are amazed at at the physical diversity that can be seen between full sisters or full brothers that are produced from the same flush. This diversity is a result of genetic differences between embryos that is created by the unique sample of parental DNA in each egg and in each sperm that are brought together during fertilisation.
Genetic differences between embryos
The use of artificial breeding technologies, including the production of embryos through flushing and artificial insemination are common-place in the Wagyu industry.
Many Wagyu breeders are amazed at the physical diversity that can be seen between full sisters or full brothers that are produced from the same flush. This diversity is a result of genetic differences between embryos that is created by the unique sample of parental DNA in each egg and in each sperm that are brought together during fertilisation.
Identical twins are genetically identical, they form from a single fertilised egg splitting early in embryo development to form two identical copies, each with the same DNA.
Flush sisters or flush brothers are not identical twins. Flush sisters or brothers are no different to normal brothers or sisters, they are genetically different versions of the sire and dam’s DNA combining. Flush sisters or brothers share the same sire and dam, like any other naturally produced sister or brother. The only different is, that they are produced at the same point in time.
Each individual flush sibling is the product of a different sperm and different egg. Each sperm and each egg caries a different sample of sire or dam’s DNA. This is why there is genetic diversity between siblings and why flush siblings are no different to normal brothers or sisters.
Figure 1
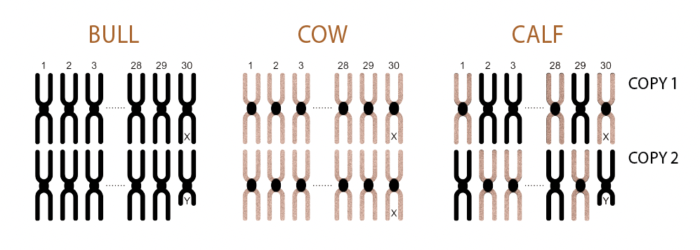
Each calf receives one copy of each of 30 chromosomes from each parent (60 copies in total – 2 of each chromosome).
Within Wagyu BREEDPLAN, flush siblings and full siblings produced through normal matings used to only have the same EBVs estimated for each sibling. These were based on the mid-parent values, basically 50% of the sire and 50% of the dam. Once performance records are added to BREEDPLAN, the EBVs for full siblings would then change to reflect the phenotype differences observed by breeders.
Now that we have Single-Step Wagyu BREEDPLAN, including genomic information (50K SNP data) on flush siblings at registration we can tell exactly which DNA came from each parent and what the relative value of this DNA for each trait is in each of the progeny.
This means that genomics in BREEDPLAN can estimate the genetic difference between full siblings (including flush siblings) early on in life so that the genetic difference between siblings can be used within management and breeding decisions on-farm. The EBVs for full siblings can vary greatly depending on the relative merit of the genes they received from the parents.
To understand how this genetic diversity occurs between siblings, we need to understand the mechanism through which the diversity is created within the sex cells (sperm and eggs) of an animal.
Genetic Material
Chromosomes are the cellular structures that maintain and transmit genetic information. They are made of deoxyribonucleic acid (DNA) combined with proteins. The DNA provides the blueprint for the physical, and some of the behavioural, traits of the animal. These traits are a unique and random mix of the parents determined by which particular sperm happens to fertilise which particular egg.
Chromosome pairs
Every cell in cattle (except the gametes) contains 30 chromosome pairs or 60 chromosomes in total. The 60 chromosomes represent one copy of each chromosome from each parent.
Sex cells – the gametes
Gametes are special cells that have undergone reduction and contain only 30 chromosomes (one copy of each chromosome). These gametes are the female egg and the male sperm that fuse together during fertilisation, restoring the 60 chromosomes in the fertilised cell and initiating the events that result in embryo development.
Creation of Sex Cells (Meiosis)
Meiosis is the process that creates the gametes within the sperm and the egg of each of the parents. Meiosis occurs in two stages. In meiosis stage 1, the two copies of 30 chromosomes are sorted into two groups. The cell nucleus then dissolves and the 30 pairs of chromosomes line up along the centre of the cell. Some pair members from each parent exchange portions of their DNA in a process called crossover that helps increase genetic diversity by creating non-identical pairs. See Figure 2.
Following crossover, one copy of each chromosome pair is pulled to one side of the cell while the other copy is dragged to the opposite side (see figure 3). This process (also called independent assortment) occurs randomly to separate the chromosome pairs to opposite ends of the cell. A gamete will therefore end up with the full 30 chromosomes, but each gamete will have one of many different combinations of chromosomes and crossover chromosomes from the original set of 60.
This reshuffling of genes into unique combinations increases the genetic variation in a population and explains the variation we see between siblings with the same parents.
The halving of the number of chromosomes in gametes ensures that, after fertilisation, the embryo will have the same number of chromosomes as the parents (30 come from each parent gamete to make the 60 in total). This is critical for stable sexual reproduction through successive generations.
Figure 2
An example of the crossing over between the pairs of chromosomes for chromosome three. The above is only to illustrate the principle and crossing over normally occurs between several of the chromosome pairs during meiosis.
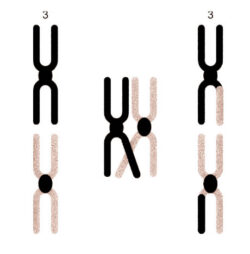
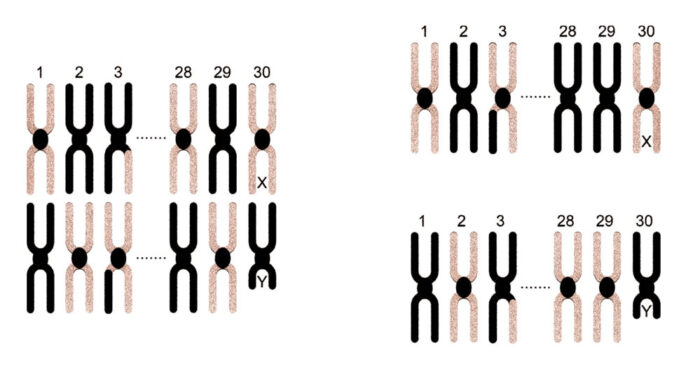
Figure 3
One chromosome of each pair is dragged to each side, halving the number of chromosomes in each cell. Independent assortment caused black chromosome 28 and (mainly pink) chromosome 3 to be dragged to the top cell.
To further genetic diversity, another round of meiosis occurs – meiosis stage 2. The chromosomes once again align at the centre of the cell. This time, the single-copy of chromosomes are distributed to two daughter cells known as gametocytes. In this way, one cell results in four non-identical gametocytes that undergo further development to become sperm or eggs.
See figure 4.
Figure 4
After meiosis finished 1 cell resulted in four non-identical sperm or egg cells.
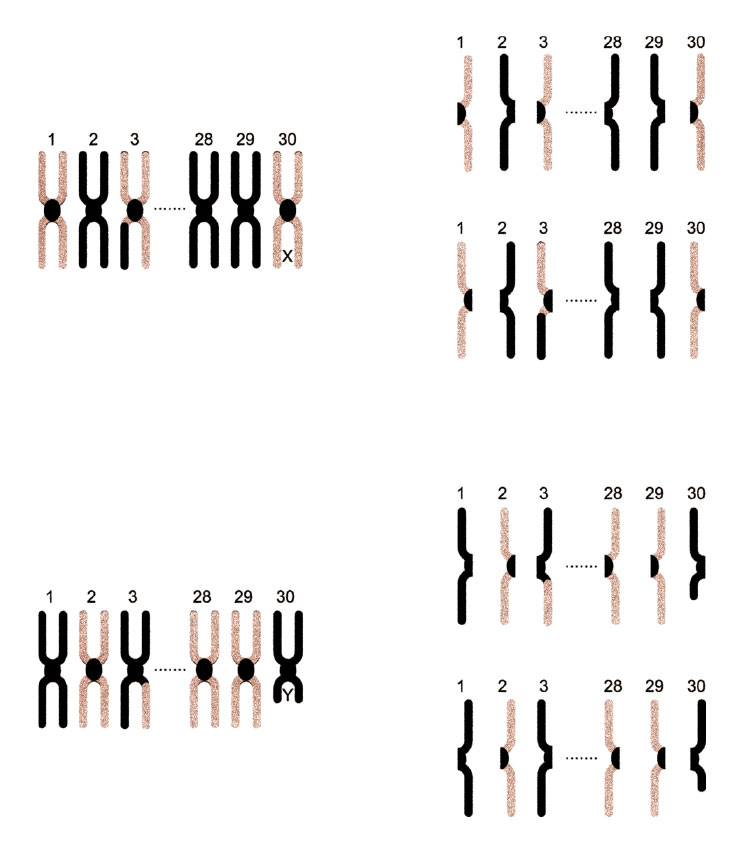
Figure 5 shows the impact of 50K genomics data on the EBVs of 7 flush siblings. In Wagyu BREEDPLAN pre-genomics, all 7 had the same Marble Score EBV, but due to weight data being provided during early life growth, the animals had different Carcase Weight EBVs.
Once 50K genomic data was added, Wagyu BREEDPLAN could determine the relative genetic merit of each individual for Marble Score, with large genetic variation now evident in this trait.
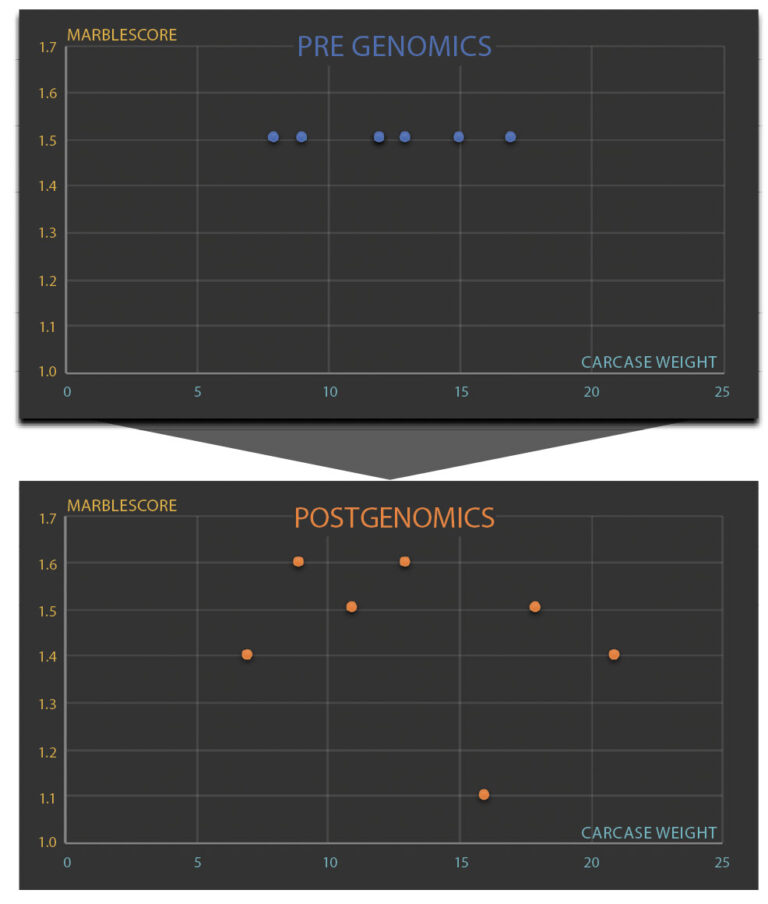
Figure 5
A story of 7 full-flush siblings – the use of genomics clearly shows the effect on EBVs.
