
Author, Dr Anneline Padayachee
Iron Riches: exploring the differences between iron derived from plant and animal foods.
World Iron Awareness week kicked off on the 28 August with the aim of raising awareness about one of the world’s most common nutrient deficiencies: iron. For most folk, iron deficiency is often linked to a health condition called “anaemia”, and anaemia is often linked to women of child-bearing age. Anaemia was first described around 400BC by Hippocrates, called it the “green disease” as it caused individuals to appear a pale greenish colour and was characterised by headaches and a desire to eat dirt (Dugan et al., 2021; King, 1996). By the 1500s, medical philosopher Johannes Lange named it chlorosis, meaning “the green disease” and linked it to “hysteria” young menstruating women seemed to experience (King, 1996). However it was not until the late 1600s that wine spiked with iron filings was used to treat “the green sickness” and remained common practice until the 1800s when tablets containing ferrous sulfate were invented (Dugan et al., 2021). And yet despite our extensive historical understanding of iron deficiency anaemia coupled with the fact that iron is the 4th most plentiful mineral in the world, iron deficiency anaemia is the most prevalent deficiency disease in the world affecting 1.2 billion people globally including children and all adults and has a direct negative impact on productivity and economic growth due to the combined effect of loss of cognitive and physical outputs (Camaschella, 2019; Horton & Ross, 2003; Zimmermann & Hurrell, 2007). Australia is not immune to iron-deficiency anaemia with 8% toddlers, 12% women, and 20% of over 85 year old individuals diagnosed with iron-deficiency anaemia (WEHI, 2023).
The basics of blood
As anaemia is a condition that affects our red blood cells (RBCs), it is important to understand the components of blood and the role they play in health. Plasma is the main component of blood at roughly 55% (CCCOnline, 2023; Informedhealth.org, 2019). It contains water, electrolytes, clotting factors, antibodies, and its primary role is to deliver nutrients, hormones, and proteins to different parts of the body as needed. It also helps remove waste, transport carbon dioxide to the lungs, and transport the blood cells (45% blood volume) and platelets (0.01%; important in preventing and stopping bleeding in a damaged blood vessel via their clotting action) through every part of the human body via the circulatory system (CCCOnline, 2023; Holinstat, 2017; Informedhealth.org, 2019). Within the blood cells category, RBCs (known as erythrocytes) account for almost 45% while white blood cells (WBCs) are only about 1% of total blood volume (CCCOnline, 2023).
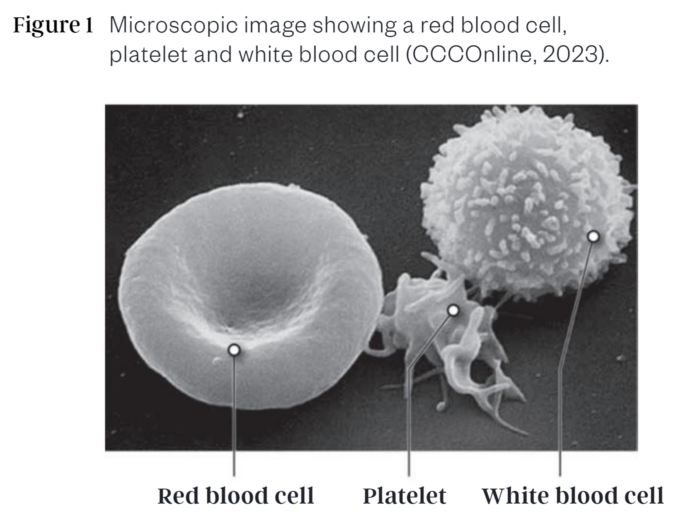
White blood cells (known as leukocytes) are created by stem cells in the bone marrow (Informedhealth.org, 2019). WBCs are commonly termed the soldier cells of the body as they are generally larger in size that RBCs, and function in the capacity of immune response. There are various WBCS that have different immune response roles (CCCOnline, 2023; Informedhealth.org, 2019):
- T-cells fight viruses
- B-cells produce antibodies that target viruses and bacteria
- Lymphocytes attack infection including detecting and killing cancerous cells
- Macrophages are the cell version of a vacuum cleaner essentially destroying bacteria, viruses, damaged cells and cell debris by phagocytosis (ingest or swallow)
- Neutrophils phagocytize foreign bodies.
Although certain lymphocytes can live for several years in the body, most WBCs live for a short time ranging from several hours to a few days (CCCOnline, 2023).
Unlike WBCs which can leave the bloodstream and enter tissues or organs that need immune assistance and are fairly colourless, RBC cannot leave the bloodstream either, live for about 120 days and gain their reddish hue from the presence of a protein molecule called haemoglobin (CCCOnline, 2023; Informedhealth.org, 2019). Given there is only one type of RBC, RBCs have one main role in the body: to carry oxygen (although it can transport a small portion of carbon dioxide back to the lungs as well). As the RBCs pass through the arteries of the lungs, the haemoglobin protein binds to oxygen molecules. Without haemoglobin, RBCs would not be able to transport oxygen. Being flattish disc shapes with a slightly concave centre, RBCs can easily change their shape to pass through narrow blood vessels as needed (Figure 1 (CCCOnline, 2023)). When the RBCs reach their intended destination, they release the oxygen molecule (Informedhealth.org, 2019). Oxygen is essential to all cells in the body as they need it to perform cellular metabolism (CCCOnline, 2023; Gopalan & Kirk, 2022). Cellular metabolism is essentially biochemical reactions needed to support cell life and include functions like creating DNA strands from nucleic acids, synthesizing proteins from amino acids, or extracting energy from foods consumed. Carbon-dioxide produced during the cellular metabolic processes is picked up by blood plasma and transported back to our lungs so we can exhale it (CCCOnline, 2023; Informedhealth.org, 2019).
What is Haemoglobin?
The essential oxygen-transportation-delivery ability that RBCs have is based on the haemoglobin molecule. Without haemoglobin, RBCs cannot transport oxygen and are in essence useless. Imagine a bus without seats; that’s what RBCs are without haemoglobin: nowhere for passengers (in this case oxygen) to sit. Haemoglobin is an iron-protein complex. It is composed of four protein molecules attached to four haem groups. Each haem group contains iron, carbon and nitrogen. It is the haem component of haemoglobin that is able to bind to oxygen. Hence a fully formed haemoglobin structure, with four haem groups is able to bind to four oxygen atoms. A single healthy RBC has about 250 million haemoglobin molecules. If you do the maths (250 million haemoglobin molecules x 4 oxygen atoms), a single RBC can carry about 1 billion oxygen atoms which is absolutely incredible for its oxygen transportation role (CCCOnline, 2023).
What is iron-deficiency anaemia?
By definition, anaemia is a health problem where an individual does not have sufficient healthy red blood cells or haemoglobin to carry and deliver adequate amounts of oxygen to all the different cellular functions taking place in the body. Anaemia can range from short term to long term, with mild or severe symptoms depending on the extent of anaemia. There are five causes of anaemia:
- Aplastic anaemia is caused by the body being unable to produce new red blood cells due to stem cells damage.
- Sickle cell anaemia is part of the genetic condition known as sickle cell disease and occurs when RBCs form curved sickle or crescent shapes that are rigid and move slowly or get stuck in blood vessels, unlike normal healthy round disc shaped RBCs which are flexible.
- Thalassaemia is a genetic blood disorder in which the body to produce less haemoglobin resulting in less oxygen being delivered by RBCs to different parts of the body.
- Vitamin deficiency anaemia is caused by low amounts of vitamin B12 and folate which lead to extra-large RBC that do not function correctly and have low oxygen carrying ability.
- Iron deficiency anaemia as the name implies, is caused by a deficiency of iron in the body results in less haemoglobin being produced and this in turn decreases how much oxygen is transported by the RBCs.
Genetics play a major role in the development of aplastic, sickle cell and thalassaemia anaemias, and hence they are less prevalent. However, nutritional iron deficiency is the most common deficiency disease globally as it is caused by physiological needs not being met from iron absorption from the diet (Camaschella, 2019; Cappellini, Musallam, & Taher, 2020; Zimmermann & Hurrell, 2007). This may be due to:
- Physiologically higher iron needs:
- During periods of rapid growth like pregnancy, infancy, childhood, adolescence
- Blood donations
- Menstrual blood loss
- Blood loss trauma from injury or illness
- High athletic demands
- Environmentally limiting conditions:
- Inadequate intake of food from poverty or lack of access
- Inadequate intake of high bioavailable dietary iron (usually associated with plant-exclusive diets)
- Malabsorption issues in the digestive tract:
- Bariatric surgery, gastrectomy, and duodenal bypass
- Helicobacter pylori infection
- Coeliac disease
- Inflammatory bowel diseases (e.g. Crohn’s disease, ulcerative colitis)
- Chronic blood loss (e.g. in the gastrointestinal tract, peptic ulcers, haemorrhoids, hookworm infestation, bowel cancers, damaged heart valves,
- Medication-related side-effects:
- Including glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs proton-pump inhibitors.
Symptoms of iron-deficiency anaemia can vary from tiredness to neurocognitive dysfunction and hair loss, and include (Lopez et al., 2016):
- Headaches
- Paleness
- Tiredness, fatigue and lethargy
- Moderate alopecia (hair loss disease)
- Restless legs syndrome
- Vertigo
- Atrophic glossitis (loss of papillae (which contain taste buds) on the tongue so that the tongue is a smooth surface)
- Dysponea (severe chronic shortness of breath)
- Dry and rough skin
- Dry and damaged/brittle hair
- Neurocognitive dysfunction
- Cardiac mumur
- Tachycardia (rapidly beating heart)
- Low birth weight infants from mothers with iron deficiency anaemia
- Stunted growth and irreversible cognitive development in infants and toddlers.
Hence iron-deficiency anaemia is a disease that can affect everyone, not just premenopausal women (Camaschella, 2019; Cappellini, Musallam, & Taher, 2020; Lopez et al., 2016; Zimmermann & Hurrell, 2007).
The role of diet iron
While iron is an essential mineral in the body, particularly in its function within the haemoglobin molecule of the RBC, the human body cannot manufacture iron. The human body does recycle iron from dead RBCs to use again, however adequate dietary intake of iron is still needed to meet physiological needs. The presence of iron in a food is one aspect of the nutrition story. The key factor when it comes to health impact is the form the iron is in. There are two types of iron derived from food: haem iron and non-haem iron. Haem-iron, as the name suggests, is found in foods with haemoglobin (i.e. blood) like animal-based foods including red meat (beef, lamb, kangaroo, goat), pork, poultry, fish (like sardines, salmon and tuna), and offal. As a general rule of thumb, the redder the meat is, the more haemoglobin it has, and hence the higher iron levels it will have. Non-haem iron is derived from foods that do not contain haemoglobin like plant-based foods including green leafy vegetables, legumes, wholegrains and fortified cereals. Eggs are unique in the sense that the egg yolk contains both haem and non-haem iron.
Haem and non-haem iron different in several ways when it comes to their absorption and nutritional characteristics (Ems, St Lucia, & Huecker, 2023; Piskin et al., 2022):
- Chemical Structure: Haem and non-haem iron have different chemical structures:
Haem iron structure is in the form of ferrous iron (Fe2+) while non-haem iron contains both ferrous iron and ferric (Fe3+) iron.
In order for iron to be absorbed by the body, it must either be in the Ferrous (Fe2+) structure or be attached to a protein molecule (like the haemoglobin structure).
Hence from a chemistry structure perspective, haem iron is more readily available for absorption that non-haem iron.
- Bioavailability (absorption):
As iron is derived from both animal and plant foods, haem and non-haem iron are both able to contribute to the body’s iron stores but at differing amounts. Haem iron is readily absorbable and relatively unaffected by the presence of other food compounds. The bioavailability (i.e. amount available for absorption after digestion) of non-haem iron is relatively high between 15-40% (Piskin et al., 2022). Conversely while plant foods contain iron, the non-haem structure requires transformation and can be affected by the presence of other food compounds (called inhibitors) resulting in less 2% – 10% bioavailability (dependent of the type of food) (Piskin et al., 2022).
- Presence of absorption inhibitors or enhancers:
Inhibitors are compounds in the food that limit/decrease absorption. In the case of haem-iron, its chemical structure combined with it being attached to protein protects it from the impact of inhibitors. This is not the situation for non-haem iron (Cappellini, Musallam, & Taher, 2020; Piskin et al., 2022; Shubham et al., 2020). Some compounds, such as phytates (found in grains and legumes), oxalates (found in spinach and beet greens), and certain polyphenols (found in tea and coffee), can inhibit the absorption of non-heme iron when consumed in large quantities. Calcium-rich foods and supplements can also interfere with non-heme iron absorption when consumed simultaneously.
As the name suggests, enhancers enhance absorption. Vitamin C is a common enhancer of non-haem iron due to its ability to convert ferric iron to ferrous iron. Non-haem iron absorption can also be increased with the addition of meat or fish to a meal. While the mechanism of action that cause this need to better understood, it is believed that the presence of certain amino acids (namely histidine and cysteine) are able to bind to iron, enhancing absorption (Hallberg et al., 2003; Shubham et al., 2020).
Although iron is one of the most common elements in the world, it is also one of the easiest to be deficient in. Iron plays many vital roles in the body, but it’s role in helping transport oxygen to every cell via RBCs is of paramount importance. While the body is able to recycle iron from dead RBCs, dietary iron is still needed to ensure adequate iron stores are maintained. Hence a lack of iron in the diet or malabsorption issues in the digestive tract is still the main cause for iron deficiency anaemia, the most prevalent deficiency disease affecting at least 15% of the global population. Chronic tiredness and lethargy to heart complications and lack of cognitive function not only impact the individual but can be also affect the productivity and economic growth of a nation. While pre-menopausal women are at a higher risk due to monthly blood loss, iron deficiency anaemia can happen to anyone at any stage of the life cycle particularly those with high needs like children, athletes, and the immune-compromised.
Iron is derived from both animal and plant-foods, however it is the structure of the iron molecule that plays a major role in the nutritional quality of the iron. Animal derived foods, including red meat like beef, contain haem iron which has an easily absorbable structure and is relatively unaffected by compounds that inhibit absorption. Red meat also provides high quality protein, which can enhance non-haem iron absorption from plant-based foods. Conversely, when it comes to iron, it is possible to obtain sufficient iron from plant-based foods, you just have to be strategic about it as the structure of non-haem iron has a lower bioavailability and is impacted by inhibitor compounds naturally present in plant foods (like phytates, oxalates, tannins, polyphenols). Combining non-haem iron sources with vitamin C-rich foods, while consuming calcium rich foods at a separate time will help to enhance iron absorption. However, if you can eat red meat (and are not following a medically advised diet), having a palm-sized portion of red meat 2-3 times per week as part of a varied, balanced diet should be encouraged as red meat iron is a nutritionally superior source of bioavailable iron.
References:
Camaschella, C. (2019). Iron deficiency. Blood, 133(1), 30-39. https://doi.org/10.1182/blood-2018-05-815944
Cappellini, M. D., Musallam, K. M., & Taher, A. T. (2020). Iron deficiency anaemia revisited. Journal of internal medicine, 287(2), 153-170.
CCCOnline. (2023). Cardiovascular Structures and Functions. In O. L. Initiative (Ed.), Anatomy and Physiology (Creative Commons Attribution-ShareAlike 4.0 International License ed.). Carnegie Mellon University. https://pressbooks.ccconline.org/bio106/chapter/cardiovascular-structures-and-functions/
Dugan, C., MacLean, B., Cabolis, K., Abeysiri, S., Khong, A., Sajic, M., Richards, T., & Collaborative, W. s. H. r. (2021). The misogyny of iron deficiency. Anaesthesia, 76, 56-62.
Ems, T., St Lucia, K., & Huecker, M. R. (2023). Biochemistry, iron absorption.
Gopalan, C., & Kirk, E. (2022). Chapter 9 – Cellular metabolism. In C. Gopalan & E. Kirk (Eds.), Biology of Cardiovascular and Metabolic Diseases (pp. 157-179). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-823421-1.00003-2
Hallberg, L., Hoppe, M., Andersson, M., & Hulthén, L. (2003). The role of meat to improve the critical iron balance during weaning. Pediatrics, 111(4), 864-870.
Holinstat, M. (2017). Normal platelet function. Cancer Metastasis Rev, 36(2), 195-198. https://doi.org/10.1007/s10555-017-9677-x
Horton, S., & Ross, J. (2003). The economics of iron deficiency. Food policy, 28(1), 51-75.
Informedhealth.org. (2019). What does blood do? Online: Institute for Quality and Efficiency in Health Care Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK279392/
King, H. (1996). Green sickness: Hippocrates, Galen and the origins of the “disease of virgins”. International Journal of the Classical Tradition, 2(3), 372-387.
Lopez, A., Cacoub, P., Macdougall, I. C., & Peyrin-Biroulet, L. (2016). Iron deficiency anaemia. The Lancet, 387(10021), 907-916.
Piskin, E., Cianciosi, D., Gulec, S., Tomas, M., & Capanoglu, E. (2022). Iron absorption: factors, limitations, and improvement methods. ACS omega, 7(24), 20441-20456.
Shubham, K., Anukiruthika, T., Dutta, S., Kashyap, A. V., Moses, J. A., & Anandharamakrishnan, C. (2020). Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends in Food Science & Technology, 99, 58-75. https://doi.org/https://doi.org/10.1016/j.tifs.2020.02.021
WEHI. (2023). Anaemia. WEHI. https://www.wehi.edu.au/research/diseases/anaemia/#:~:text=Anaemia%20is%20a%20common%20condition,over%2085%20years%20are%20anaemic.
Zimmermann, M. B., & Hurrell, R. F. (2007). Nutritional iron deficiency. The Lancet, 370(9586), 511-520. https://doi.org/https://doi.org/10.1016/S0140-6736(07)61235-5
This article is property of the Australian Wagyu Association, you must seek approval before republishing.
Media Contact: Emily Rabone (AWA Communications Manager), 0437 388 481 or [email protected]
